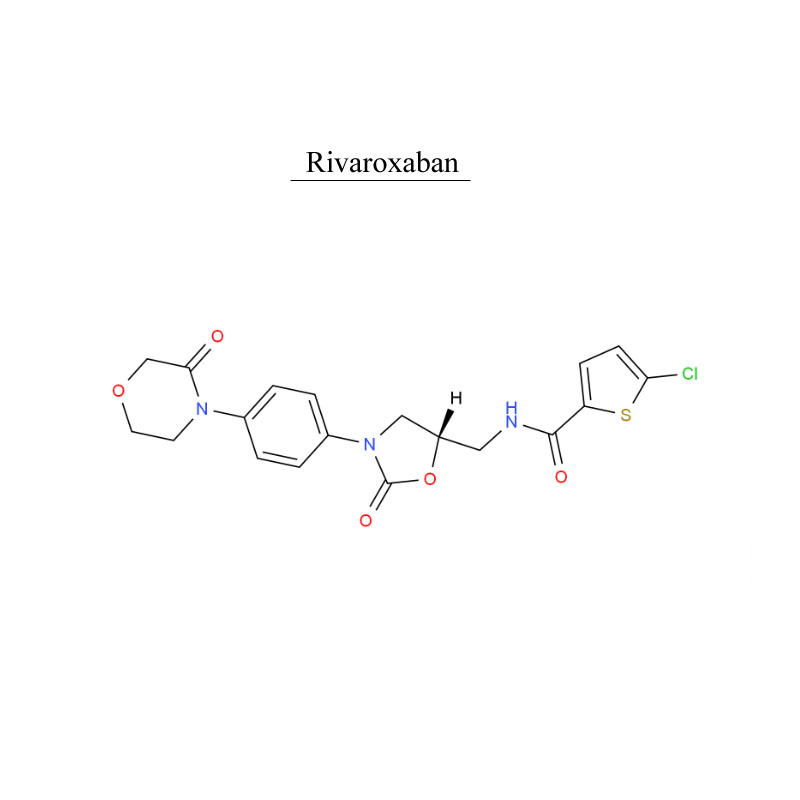
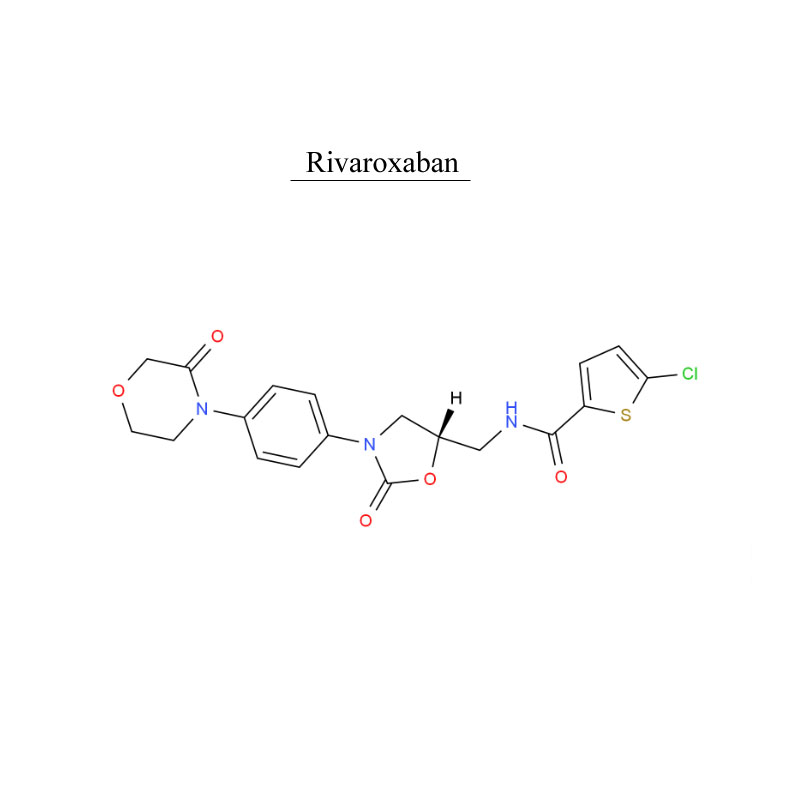
Rivaroxaban 366789-02-8 Ntshav system tiv thaiv Antithrombosis
Kev Them Nyiaj:T / T, L / C
Khoom Keeb Kwm:Tuam Tshoj
Shipping Chaw nres nkoj:Beijing / Shanghai / Hangzhou
Muaj peev xwm ntau lawm:200kg / hli
Order (MOQ):25kg ua
Lub Sijhawm Lead:3 Hnub Ua Haujlwm
Kev cia khoom:Khaws rau hauv qhov chaw txias, qhuav, chav sov.
Cov khoom siv pob:nruas
Pob loj:25kg / nruas
Cov ntaub ntawv kev nyab xeeb:Tsis yog khoom txaus ntshai
Taw qhia
Rivaroxaban, yog ib qho tshuaj anticoagulant (ntshav thinner).Nws siv nyob rau hauv cov neeg laus uas tsis yog-valvular atrial fibrillation (tshwj tsis yog atrial fibrillation vim rheumatic valvular heart disease, thiab atrial fibrillation tom qab hloov lub plawv valve) kom txo tau txoj kev pheej hmoo ntawm mob stroke thiab systemic embolism.
Rivaroxaban, yog siv los kho thiab tiv thaiv cov ntshav txhaws.
Rivaroxaban, yog siv rau cov neeg laus cov neeg mob uas tau xaiv lub duav los yog lub hauv caug hloov phais kom tiv thaiv venous thrombosis.
Specification (EP)
| Yam khoom | Specification |
| Qhov tshwm sim | Dawb los yog daj hmoov hmoov |
| Solubility | Xyaum insoluble hauv dej, dawb soluble hauv DMSO, xyaum insoluble hauv anhydrous ethanol thiab hauv heptanes. |
| Kev txheeb xyuas | IR: Spectrum yuav tsum ua raws li WS |
| HPLC-RT ntawm cov qauv nyob rau hauv enantiometric purity test yuav tsum ua raws li WS. | |
| Dej | ≤ 0.5% |
| Sulphate tshauv | ≤0.1% |
| Poob rau ziab | ≤ 0.5% |
| Cov hlau hnyav | ≤20ppm |
| Enantionmer | impurity A: ≤0.4% |
| Residual kuab tshuaj | Ethanol: ≤5000ppm Ethyl acetate: ≤5000ppm Acetone: ≤5000ppm N, N-Dimethylformamide: ≤880ppm Methylene chloride: ≤600ppm Benzene: ≤2ppm |
| Muaj feem xyuam | impurity B: ≤0.10% impurity D: ≤0.10% impurity E: ≤0.10% impurity F: ≤0.10% impurity G: ≤0.10% impurity H: ≤0.10% impurity I: ≤0.10% impurity J: ≤0.10% RVB-4-NJSH: ≤ 0.10% RVB-ZA: ≤0.10% RVB-BAM: ≤0.10% RVB-ZC: ≤0.10% RVB-HBY: ≤ 75ppm Lwm yam tsis paub impurity: ≤0.10% Tag nrho impurity: ≤0.3% |
| Particle loj faib | D10:/ D50:/ D90:/ |
| Assay (anhydrous khoom) | 98.0-102.0 Nws |